Food Facility Register
Owners or operators of establishments involved in the production and distribution of food intended for consumption in the United States (US) must register with the FDA and renew their registration anually:
FDA-FOOD FACILITIES REGISTER. SECTION 415 DE LA LEY FD&C
REGISTER HEREFDA-FOOD CANNING ESTABLISHMENT REGISTRATION – FCE/ SID 2541
REGISTER HERE
Labeling
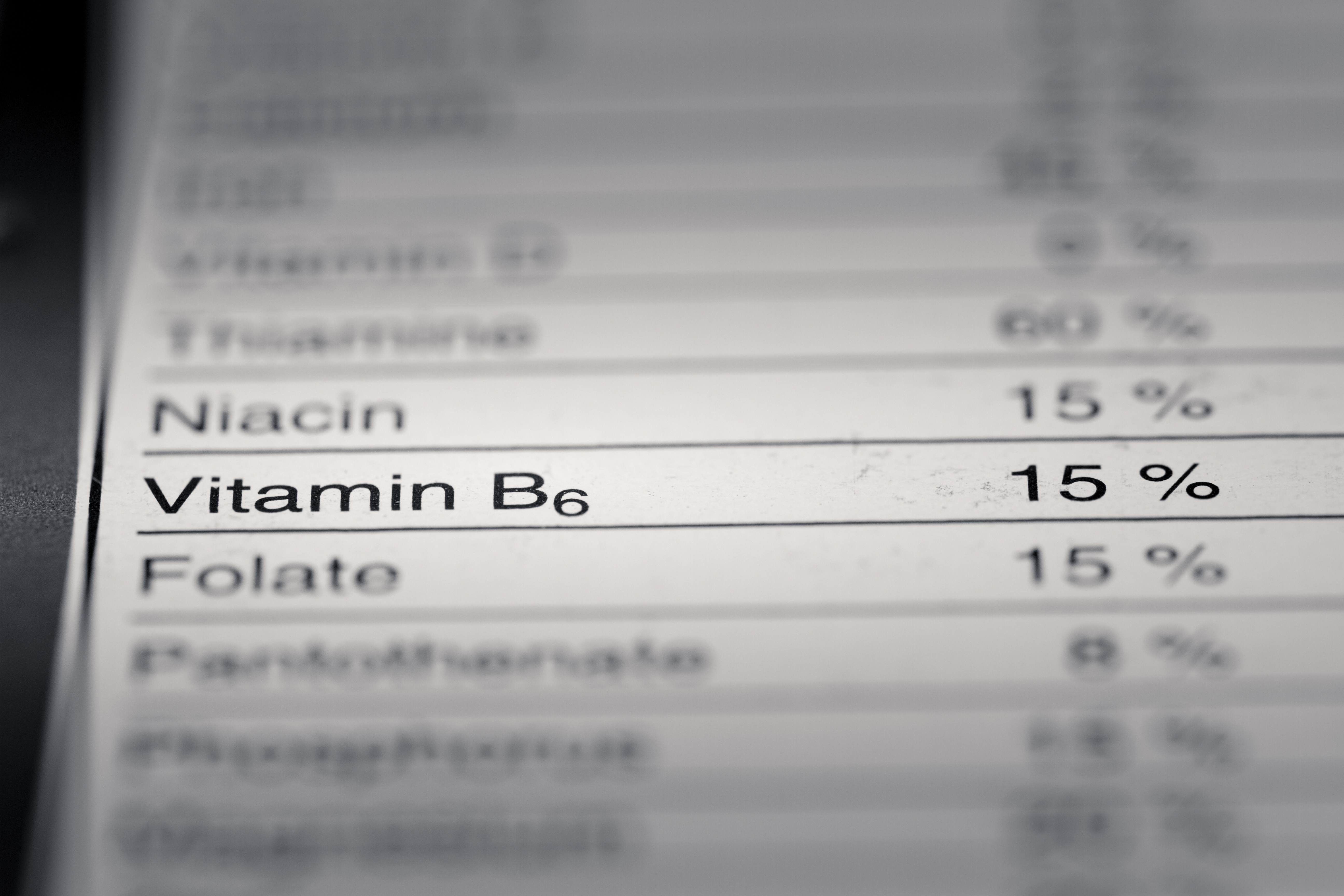
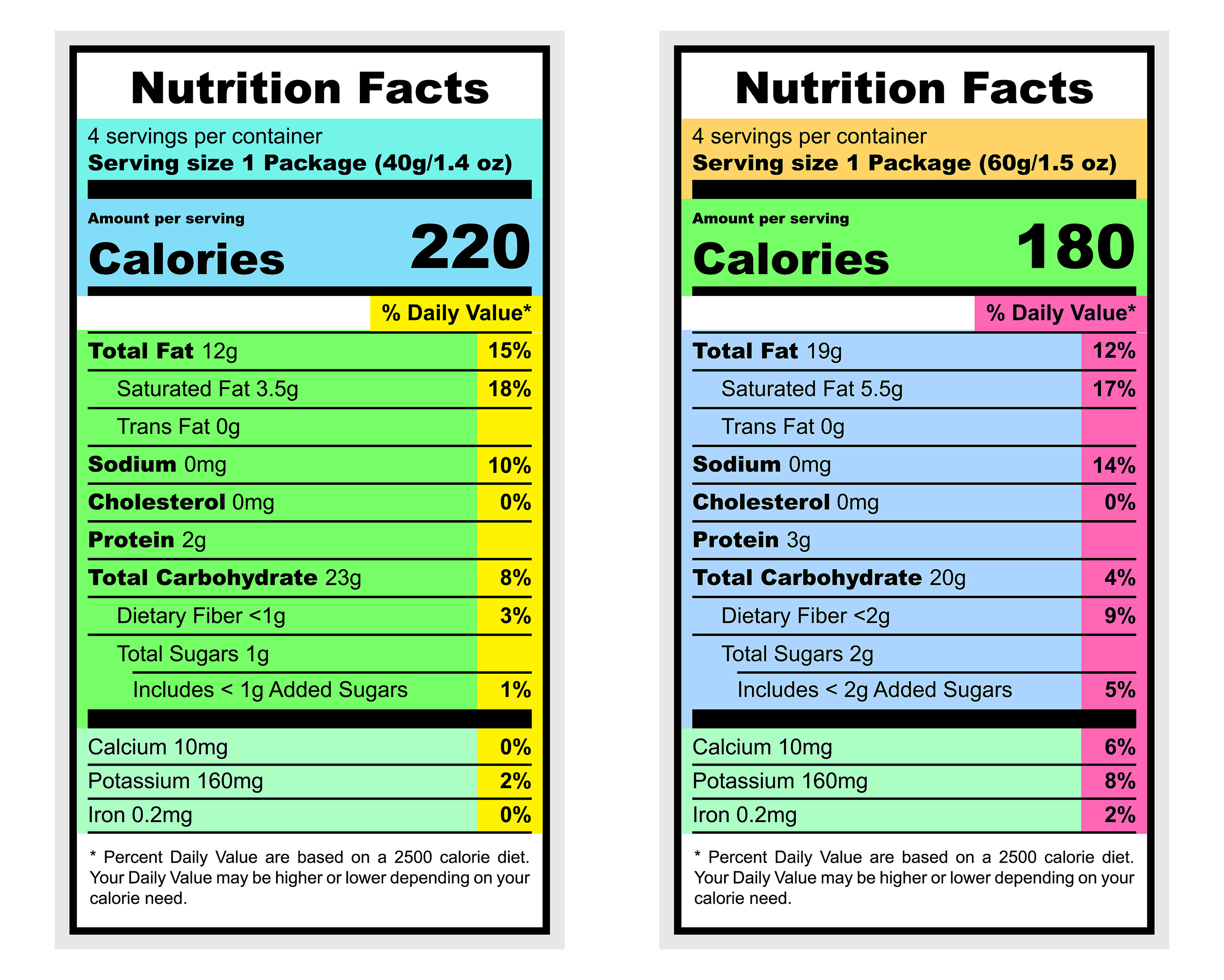
For dietary supplements, you must place all required statements on the label, either on the front label panel (the main display panel) or on the information panel (usually the label panel immediately to the right of the label panel). main display, as seen by the consumer when in front of the panel). product), unless otherwise specified in the regulation (i.e., exemptions). 21 CFR 101.2(b) and (d), 21 CFR 101.9(j)(13) and (j)(17), 21 CFR 101.36(g), (i)(2) and (i)(5)
Regulatory technical report with the following information:
- Identity statement
- Net package content
- Contact information
- List of ingredients
- Nutritional information
Regulatory technical report with the following information:
- Identity statement
- Net package content
- Contact information
- List of ingredients
- Nutritional information
Prior notice
On May 5, 2011, the FDA published an interim final rule requiring a person submitting a prior notice for imported food, including animal food, to report the name of any country to which the item was denied entry.
FDA-PRIOR NOTICE
Part 1, Subpart I. Prior Export Notification
Package of services for shipments
1 BOARDING
10 SHIPMENTS
20 SHIPMENTS
50 SHIPMENTS
Technical support
Import alerts inform FDA field staff and the public that the agency has sufficient evidence to allow detention without physical examination (DWPE) of products that appear to violate FDA laws and regulations. These violations may be related to the product, manufacturer, shipper, and/or other information. Before importing into the United States, importers must know if their products are subject to DWPE.
FDA Import/Unlock Alert